Document Center
Deep UV LED
Product trends
Establishment of product document library on February 15, 2020
March 17, 2020 new 3MW monochromatic lamp beads on the market
On June 15, 2020, the name of deep purple scientific and technological products will be adjusted significantly
August 17, 2020: Shenzhen purple Technology Co crystal lamp bead products on the market
Product introduction
quick get start
Practical cases
exception handling
common questions
What is the effective irradiation distance of uvc-led?
Does deep UV uvc-led produce ozone during operation?
Does UVC have harm to human body and how to protect it?
How to choose the window material for deep UV LED applications?
Can deep ultraviolet decompose formaldehyde?
How far is the propagation distance of uvc-led in water?
Effective sterilization range of deep UV uvc-led?
How to test the luminous angle of lamp bead quickly?
Five factors affecting the disinfection or sterilization effect of deep UV LED
Why does uvc-led have color difference?
Is uvc-led driven by constant voltage or constant current?
Can deep ultraviolet decompose formaldehyde?
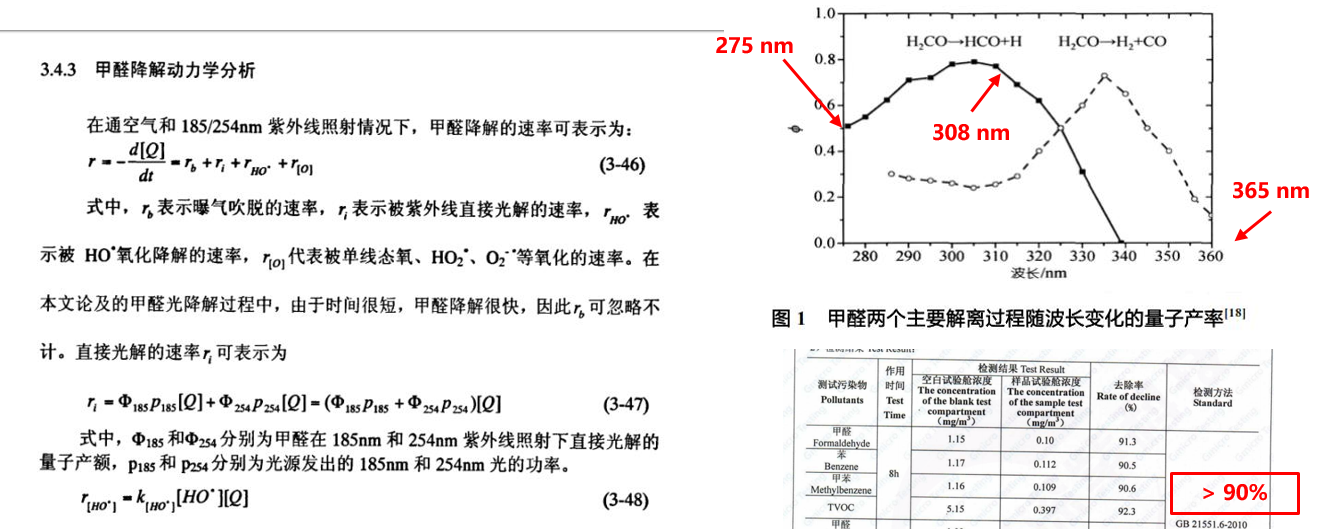
There are two kinetic processes in the decomposition of formaldehyde by UV: one is that the photolysis is interrupted by UV directly; the other is that the UV reacts with water in the air to produce OH radical to decompose formaldehyde. (mercury lamp tube is OK). Deep ultraviolet can directly decompose formaldehyde and other organic pollutants by breaking the chemical bond of formaldehyde.
Can deep ultraviolet ray decompose formaldehyde and other organic pollutants? In fact, many people think that it should not work, but in fact it can. The bond energy of O-O bond is 5.1ev corresponding to 243nm, but the necessary C-H bond energy in organic compounds is 4.31ev, corresponding to 283nm, C-O bond energy is 3.39ev, corresponding to 366nm. If the photon energy of ultraviolet is higher than the bond energy, it can be interrupted, just as it destroys DNA molecular chain. Therefore, UV can decompose formaldehyde.
However, we should also note that the decomposition process of formaldehyde is very complicated, and its two decomposition processes are directly related to the wavelength. The quantum yield changes of formaldehyde decomposition at different wavelengths. Although we don't know what two peaks are at 305 nm and 340 nm, there is no doubt that UV can decompose formaldehyde by itself. Compared with toluene and TVOC, UV can decompose.
